
Methamphetamine (meth) is the number one killer in San Diego County among those using drugs, and a significant driver of violence in the community. Nationwide, meth is the fastest growing drug of abuse, representing over 798,000 annual emergency department (ED) visits, with deaths increasing 14-fold since 2015 (32,856 deaths in 2021 alone), yet no therapeutics are available. There is an urgent need for a safe rapidly acting antidote for methamphetamine.
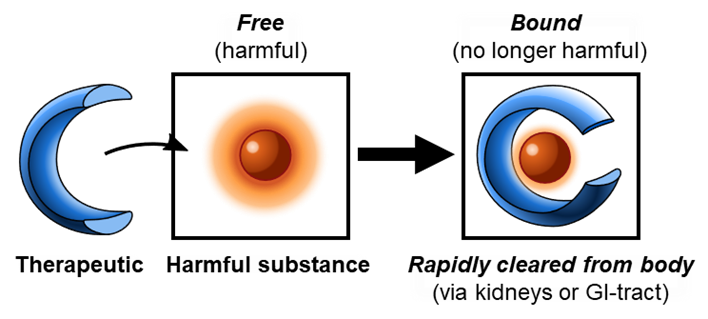
With support from the National Institute on Drug Abuse (NIDA), Cambridge, MA-based Clear Scientific (www.clearsci.com) is developing an antidote for meth for use in the ED. This small molecule therapeutic, CS-1103, reverses meth intoxication by sequestering it in the plasma compartment and removing it from the central nervous system effect site. The now ‘inactive’ meth is eliminated from the body by filtration in the kidney. Rapid sequestration and clearance of meth reverses its effects. This mechanism of action is similar to Sugammadex (BRIDION®), a reversal agent for neuromuscular blockade. Unlike antibody-based treatments, CS-1103 not only binds harmful substances, but also rapidly removes them from the body – “Remove the Cause, Remove the EffectTM”. CS-1103 is formulated for intravenous injection for use in the ED.
In nonclinical studies, CS-1103 was highly effective in lowering the level of meth and rapidly reversing its toxic effects. CS-1103 has an excellent safety profile in Good Laboratory Practice (GLP) studies with rapid clearance and has been produced at scale under Current By Clear Scientific Good Manufacturing Practice (cGMP).
It is anticipated that the Phase 1 and 2/3 Clinical Trials will be completed in 2023 and 2024, respectively, with availability to ED physicians by late 2024.
For more information, please listen to the discussion between Dr. Roneet Lev and the Clear Scientific team on the High Truths podcast [Episode 91 September 19,2022].
